
A rapid LC–MS/MS method is presented for the quantitative analysis of 16 cannabinoids in hemp samples with a single dilution protocol.

A rapid LC–MS/MS method is presented for the quantitative analysis of 16 cannabinoids in hemp samples with a single dilution protocol.

This article focuses on the use of GC–MS for the analysis of derivatized cannabinoids in hempseed oil matrix.

A review of the chemical makeup of cannabis extracts and how they affect the human body. From terpenes to cannabinoids, this article takes a deep dive into the chemistry of cannabis extracts.

An interview with an amazing family of four doctors—the Knox Docs—who are key opinion leaders in cannabis therapeutics, endocannabinology, and cannabinoid medicine.
A closer look at some of the current in vitro evidence suggesting that cannabinoids possess potent activity against a range of gram-positive bacteria.

Dr. Linda Klumpers discusses her interest in neuroscience, her most significant work, success stories from Cannify—an online science-matching tool—and more.

This study highlights a potential concern for the quantitation of acid phytocannabinoids.

A large data set from Washington (140,000 flower samples) is scrutinized here for evidence of consistently propagated strains with higher than a 2-to-1 ratio.


Applications of a quantitative mid-infrared spectrometer for hemp farmers, hemp extractors, state regulators, and law enforcement are discussed.

A review of the literature to examine the analgesic properties of cannabis.

Dr. Andrea Holmes discusses how she’s using colorimetric analysis to discover novel minor cannabinoids, the potential role of smartphone compatibility in detection, what’s next for cannabinoid research, and more.
This article reviews state-of-the-art approaches to produce cyclodextrinphytocannabinoid complexes, related processing and analytical techniques, and implications for cannabis product development.

This review article from an experienced pharmaceutical microbiologist discusses the risks of microbial contamination for the full range of cannabis-derived products and recommends the most appropriate microbiological quality requirements for each product.

Better, accurate and more reliable analytical testing is needed in the industry. The author discusses one such method that goes well beyond the current contaminant testing done today in most laboratories.

Some hemp-based products are mislabeled regarding their level of CBD. The authors explain why and how that happens.

Here we review the current and pertinent literature in utilizing cannabis derivatives in animals and discuss the future forecast of cannabis in veterinary medicine.
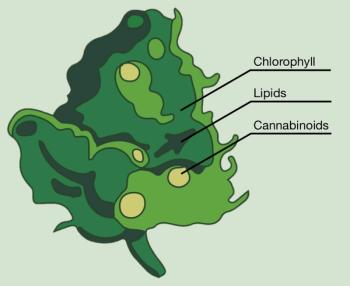
At a glance, modern extraction machines can seem a little mysterious: plant material is added to an extraction chamber, processing parameters are chosen, the extraction process is carried out, and an output of material is collected. Part I of this series examines the two main biologically-inherent starting-material influences.
Laboratories are challenged with highly regulated and difficult sample schematics, sample preparation, extraction, and testing procedures that try to ensure accuracy and precision of testing. Accuracy in analytical testing starts at the very beginning with sampling and sample preparation prior to testing.


Josh Blacher, Chief Business Officer at InMed Pharmaceuticals, recently spoke to us about the groundbreaking researching his company is doing using biosynthesis to create cannabinoid-based pharmaceutical products.

A look at how TD-GC–MS analysis eliminates conventional sample preparation regimes and can be used as a good rapid screening technique.

Here the LC–UV separation of 16 cannabinoids of interest was performed while the potential impact from minor cannabinoids and terpenes on reported potency values was monitored.

Reference standard qualifications are discussed and techniques for qualifying incoming reference standards are suggested.
