
How can cannabis businesses start formulating like the pharmaceutical industry and why it’s important to do so.

How can cannabis businesses start formulating like the pharmaceutical industry and why it’s important to do so.

Isolated CBD, full-spectrum oil, and even smokable hemp are all being diluted down by a draconian regulatory framework that needs to be changed to facilitate growth, innovation, and prosperity throughout the industry.

As globalization of demand for cannabis and its derivative products increase, new technology and artificial intelligence solutions can greatly boost the output of production and processing.

Here, we take a closer look at buffers to see what solutions they might offer.

A review of the general state standard quality control checkpoints and how companies can go above and beyond by applying federal requirements to their businesses.
Here we discuss issues such as how a repeatable outcome is a critical component of developing cannabis as a reliable medicinal treatment, insight into how to work out extraction goals such as quality terpene development, and more.

CBD is a growing industry. In this article, topicals and their growing popularity are discussed.

How can cannabis enterprise resource planning software help organize your data?

How do falling films work and what are potential solutions to improve them?

This article proposes cannabis labeling standards to serve as a foundation for the regulation of cannabis product packaging for both medical and adult-use products.

In this article, you will learn about the GxP requirements for laboratories, production facilities, and distribution of cannabis.

The industry needs to address energy efficiency, plastic use, and wastewater to build a long-term reputation of accessibility, legitimacy, and sustainability.

An explanation of the causes of potency loss in infused beverages from either a chemical or physical route.

A look at some important questions that business owners must consider during the initial design phase when starting an extraction facility.

This tutorial article outlines a holistic approach to scaling up.
This article reviews state-of-the-art approaches to produce cyclodextrinphytocannabinoid complexes, related processing and analytical techniques, and implications for cannabis product development.

The final installment of this series reviews how processing parameters affect yields in extraction.

This review article from an experienced pharmaceutical microbiologist discusses the risks of microbial contamination for the full range of cannabis-derived products and recommends the most appropriate microbiological quality requirements for each product.

An explanation on the theory and measurement of water activity, its mode of action for microbial control, existing water activity regulations for cannabis, and more.

There are many ways to ingest tetrahydrocannabinol (THC). But more innovative methods are being explored, with interesting results for the consumer.
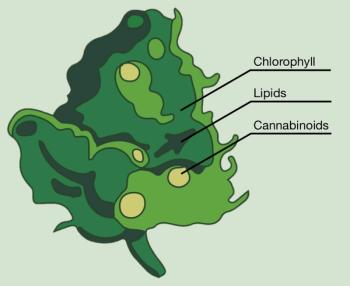
At a glance, modern extraction machines can seem a little mysterious: plant material is added to an extraction chamber, processing parameters are chosen, the extraction process is carried out, and an output of material is collected. Part I of this series examines the two main biologically-inherent starting-material influences.

This tutorial offers a broad overview of lean manufacturing.

Which formulation choices will support safety, efficacy, stability, and consumer acceptance.

George Anastassov, the CEO of AXIM Biotech, recently spoke to us about the current good manufacturing methods (cGMPs) his company has developed, their significance to the market, and plans for the future.
