Understanding Sources of Heavy Metals in Cannabis and Hemp Consumer Products, Part II: Is the Fractured Nature of State-based Regulations Ignoring the Evidence?
The second part of this column will take a look at specific examples in the public domain of elemental contamination of cannabis consumer products, which shows that other elements are worthy of consideration and should be a part of the regulatory framework.
In this month’s column, guest contributor, Rob Thomas takes a critical look at the big four heavy metals which are regulated by the vast majority of US states and suggests that there is compelling evidence in the public domain that the panel should be increased because there is not a good understanding of the sources of elemental contaminants in the production of cannabis consumer products. As a result of the inadequate nature of these state-based regulations, consumer safety is most likely being compromised.
Part I of this series examined the fractured nature of state-based regulations and compared it with the federal limits for pharmaceutical products and dietary supplements. Part II, will take a look at specific examples in the public domain of elemental contamination of cannabis consumer products, which shows that other elements are worthy of consideration and should be a part of the regulatory framework.
Standardized Analytical Methods
Both AOAC International (AOAC) and ASTM International (ASTM) have developed standardized methods for carrying out the measurement of heavy metals in cannabis matrices by inductively coupled plasma mass spectrometry (ICP-MS). The AOAC (OMA 2021.03) surveillance method for the routine monitoring of 12 elements, including lead (Pb), cadmium (Cd), arsenic (As), mercury (Hg), antimony (Sb), barium (Ba), chromium (Cr), copper (Cu), nickel (Ni), silver (Ag), selenium (Se), and zinc (Zn) was first published in 2021 (1), while ASTM International's D37 Cannabis Committee (Under D37.03) recently developed a more detailed standardized ICP-MS method (ASTM D8469-22) for up to 23 elemental contaminants in different cannabis matrices in 2023 (2). As part of the ASTM validation procedure, an interlaboratory proficiency testing study was carried out where four volunteer laboratories were asked to test three different samples for a panel of toxic elements based on interest expressed by the cannabis community for safety and regulatory reasons. The three samples were a control plant material, National Institute of Standards and Technology (NIST) 1575a pine needles, together with a National Research Council of Canada (NRC) Hemp 1 and a NIST Hemp 4 reference material (described in NIST CannaQAP Exercise 2 Program) (3). Every test result represents individual determinations, and all participants were instructed to report three replicates for each material. The spike recoveries, precision, bias, reproducibility and uncertainty of the test method was carried out according to Practice E691 defined in ASTM Research Report No. RR:D37-2001 (4). The values obtained by four independent testing laboratories were averaged together with their interlaboratory standard deviations (SD) and compared with the values obtained by the respective standards organizations. Table I shows the full panel of elements for one of the hemp reference materials (NIST 4 CannaQAP material) together with standard deviation (reproducibility) of the results.
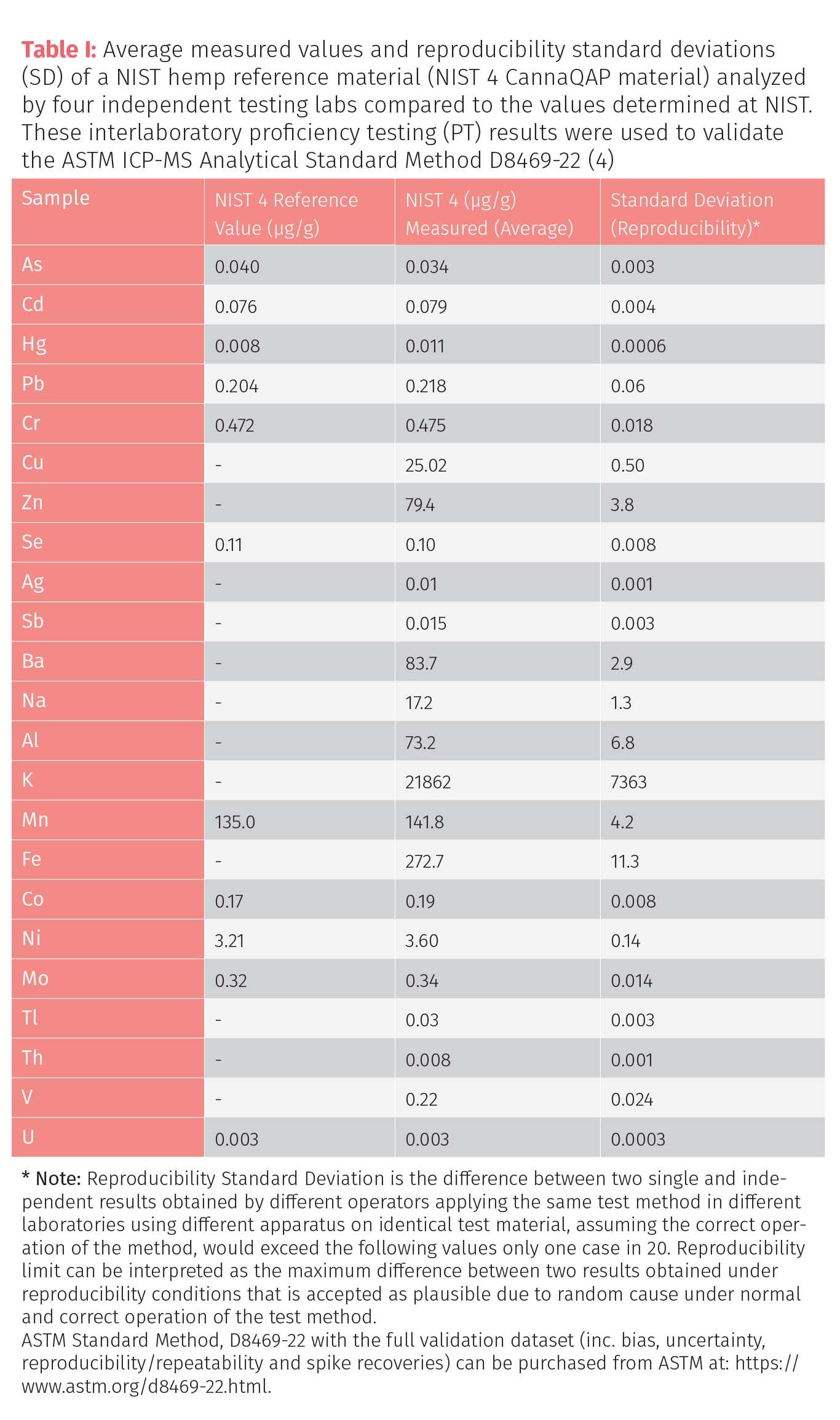
It is interesting to note that while only 12 toxic metals were studied in this material by NIST, 11 additional elements have been measured and quantified meaning that if this hemp sample is typical, it contains toxic metals which would escape the scrutiny of most state regulators. However, if there is a positive, it demonstrates how matrix-based reference materials can offer testing labs the flexibility to select the heavy metals that are important in their jurisdiction and in particular to encourage state regulators to expand the panel beyond the big four to those additional metals that are worthy of regulatory oversight. At the time of writing this article, this NIST Hemp 4 reference material is nearing completion. After the CannaQAP Exercise 2 was completed, several modifications were made to the material before it was homogenized and packaged, and as a result, values in this publication will not be the final assigned values. However, the material along with the values in a Reference Material Information Sheet, became available in the summer of 2024 at https://shop.nist.gov.
Routine Screening of Commercially Grown Cannabis and Hemp
At an ASTM D37 symposium in October 2023 dedicated to the measurement of contaminants in cannabis and hemp consumer products, compelling data was presented by several researchers which suggested that 15-20 elemental contaminants could be worthy of consideration (5). One of the presentations was by Kaycha Labs (a private laboratory that conducts routine screening of cannabis products in Colorado) in collaboration with researchers at the Colorado School of Public Health, Environmental, and Occupational Health, Center for Health, Work & Environment. The title of their talk, “Preliminary Levels and Distribution of Health-Relevant Heavy Metals in Commercially Grown Cannabis and Hemp,” characterized levels of 20 heavy metals in 60 Colorado market cannabis flower using newly validated laboratory methods. Flower samples were randomized, de-identified and separated by type of grow operation (indoor/outdoor). Additionally, to ensure characterization of those samples that may pose the greatest risk of heavy metal-related health effects, the random sample also included samples of cannabis flower that failed Colorado heavy metal limits for Pb (0.5 ppm), Cd (0.2 ppm), Hg (0.1 ppm), or As (0.2 ppm).
The study was extensive and represented one of the most comprehensive characterizations to date of monitoring a wider panel of elemental contaminants beyond the currently regulated “big four” metals of arsenic, cadmium, lead, and mercury. Moreover, the conclusion of the study was very revealing as evidenced by the following statement (6), “Given the potential for additional accumulation of metals from methods of cannabis consumption, particularly vaping, and the wide range of systemic health impacts associated with heavy metal exposure, further resources should be allocated to considering the expansion of current regulatory requirements of these products. Changes in regulatory testing requirements should be accompanied by investments in public health laboratory infrastructure and workforce investments to support expanded testing.” This study was written up and is a part of the symposium proceedings which is currently moving through the publication process and is expected to be published in the ASTM Journal of Testing and Evaluation (JTE) in the Fall of 2024 (6).
Production Related Contamination
There is also a great deal of data in the public domain of specific heavy metals being found not only from the cultivation and growing environment but also in production of cannabis and hemp consumer products related to corrosion of metal-based equipment, metallic components and storage vessels containing metals, that are in contact with liquids, oils and extracts during the cannabinoid manufacturing, production, packaging, and delivery process. For example, lead is one of the most common adulterants because it has been so widely used over the years in our everyday lives including water pipes, paint, gasoline, ceramic glazes, alloys, solders, etc., and is known to cause neurological issues, severe nerve damage, cardiovascular problems, which greatly increase the incidence of certain cancers. There are many cases of lead and other toxic metals finding their way into commercially available cannabinoid consumer products, but let’s take a closer look at some specific cases (7).
Lead in CBD Tinctures
Tom Gluodenis PhD, Professor, Dept of Chemistry & Physics, and coworkers at Lincoln University carried out a trace metal profiling study of cannabidiol (CBD) tincture oils which were manufactured by US Hemp Authority certified companies with the expectation that the products would represent the highest quality available (8). 15 samples of CBD tincture oil were purchased, each from a different manufacturer, while two additional purchases for a total of three samples per manufacturer, were made at six month increments so that “lot to lot” variations could be assessed for each manufactured product. In an attempt to minimize experimental variables, each product purchased was a full spectrum tincture oil (containing all cannabinoid extracts including <0.3% tetrahydrocannabinol [THC]). In addition to the “certified samples”, a random sampling of 10 additional products purchased locally at gas stations, smoke shops, and other outlets were analyzed for comparison purposes.
The oils were digested in a mixture of nitric and hydrochloric acid in a microwave digestion oven and analyzed by ICP-MS. Most of the samples were very clean and were below the regulated limits for the four heavy metals required by the state of Pennsylvania. However, four of them contained Pb levels which were all higher than the 0.5 ppm regulatory limit for Pennsylvania. Moreover, the three different lots for one of the “certified samples” and one of the “shop-bought” samples showed significant differences as seen in Table II, suggesting that the Pb contamination was variable and predictable and could have been caused by a source of lead in the production process or storage equipment.
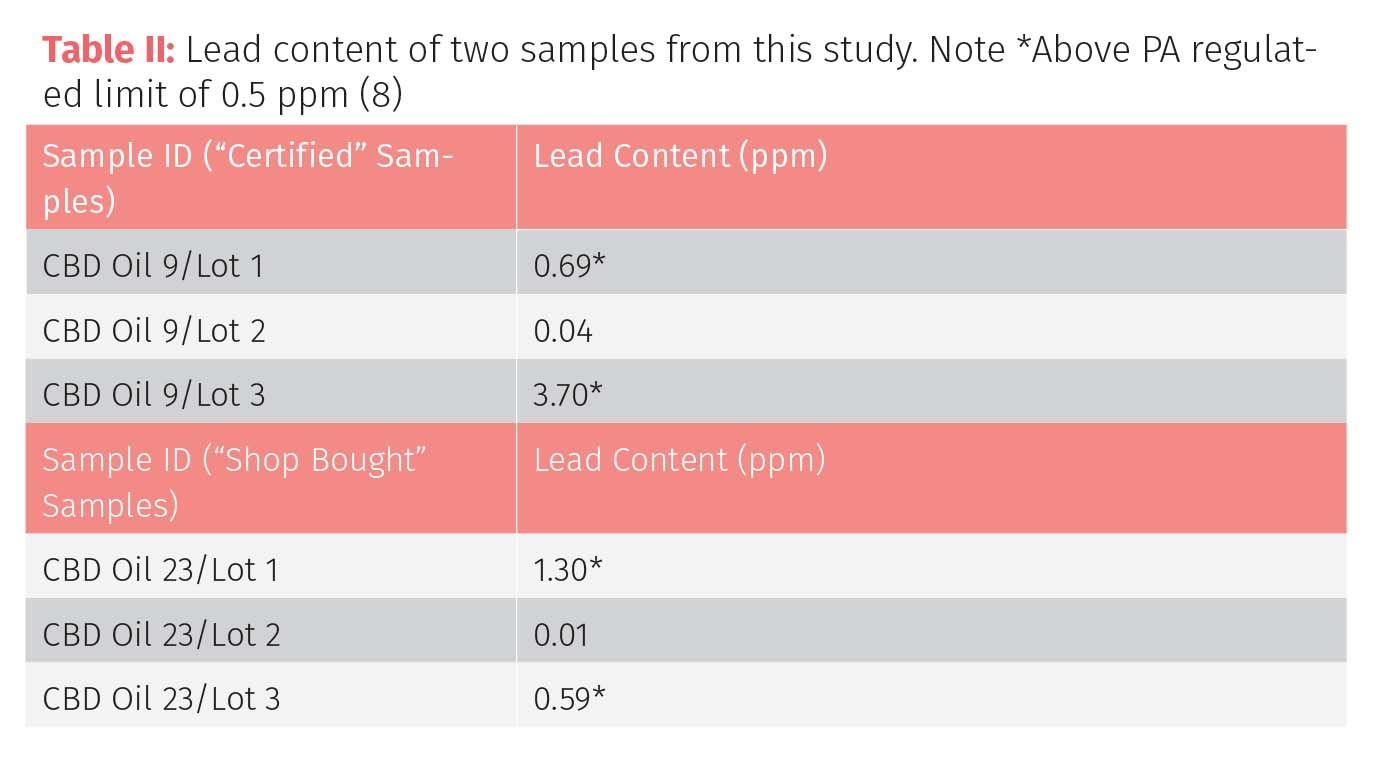
Interestingly, the study also confirmed that the two samples were in excess of 100 µg/g (ppm) tin (Sn), which would suggest that tin-based solder was the source of the contamination. This is below the ICH Q3D PDE limit for oral drug products of 600 µg/g. However, it is worth noting that this limit is based on inorganic Sn, whereas Sn toxicity is heavily dependent upon the speciated form. For example, some organotin compounds have been shown to result in renal and neurotoxicity, which is largely due to the lipophilic nature of organotin compounds allowing them to be readily absorbed from the gastrointestinal tract where they can then migrate to other parts of the body (9). However, the form of the tin present in the CBD oils is currently unknown and there have been no known speciation studies conducted to date in these types of samples.
Lead-Contaminated Dropper Bottles
Diane Picket, Chief of Florida Department of Agriculture and Consumer Services Food Safety Lab and co-workers at the Florida Department of Agriculture Consumer Services Food Safety Lab (FDACS) did an exhaustive study of monitoring a group of toxic metals in 208 hemp extract oils (10). The good news was that lead was the only heavy metal that was found in appreciable amounts. 42 of the samples tested positive for Pb, but below the regulatory limit for of 0.5 ppm. However, the bad news is that 10 of the samples exceeded the 0.5 ppm limit, with two of them being over the Environmental Protection Agency (EPA) Resource Conservation and Recovery Act (RCRA) limit for hazardous waste of 5 ppm (10x regulated limit for Florida [FL]) while one of them was 19 ppm (38x higher than the legal limit). On further investigation of some of the samples being stored in glass bottles they found that the lead levels increased over time, which suggested the Pb was either being leached out of the glass or the ink used for the graduated marks. They haven’t ruled out the borosilicate glass dropper bottles being the culprit because unless it is high purity, some glasses are known to contain transition and heavy metals contaminants from the glass manufacturing process. However, their study is still on-going, but it now seems more than likely that the ink used for the graduated marks is a Pb-based formulation similar to leaded household paints that were eventually banned in the US in 1978.
Metal Dyes Used in Cannabis Rolling Papers
One area that has received little attention is the possible exposure to potentially hazardous levels of toxic elements from cannabis rolling papers. The elemental composition of rolling papers has gone mostly under the radar, with the exceptions of some states that implement regulations only when they are part of a final product such as cannabis pre-rolls. Well, this all may be about to change with a fascinating study in the journal, ACS Omega, by researchers at Lake Superior State University (LSSU) entitled, “Elemental Composition of Commercially Available Cannabis Rolling Papers” (11). In this study, the concentrations of 26 elements in commercially available rolling papers are characterized and measured by ICP-MS and Scanning Electron Microscopy (SEM) combined with Energy Dispersive X-ray Spectroscopy (EDXS) to estimate potential maximum exposures limits relative to dosages in drug inhalation products as defined in United States Pharmacopeia (USP) Chapter 232 and ICH Q3D Guidelines. Exposure estimates indicate that the concentrations of several elements, including Ag, Ba, Cr, Cu, molybdenum (Mo), Ni, Sb, titanium (Ti) and vanadium (V), are likely present at elevated quantities in some papers due to product design and manufacturing processes. In particular, Cu-based pigments are used by many of the manufacturers and that regular use of these products might result in exposures up to 5-10 times the maximum USP/ICH limits for inhalation drugs. The overall conclusion of the publication suggests that cannabis users may be inhaling unsafe levels of toxic metals as rolling papers burn, particularly those that contain dyes or have metallic-colored tips. These metals are not typically required by most states, which is further justification to expand the regulated panel beyond the big four heavy metals (Pb, Cd, As, Hg) to ensure consumer safety.
Metallic Particles in Vaping Carts
It is estimated that vaping accounts for almost 50% of all cannabis consumption today. So, any information in the public domain about vaping and the potential for contaminants in the vape extract is of critical importance. Unfortunately, we know that many of the components used in today’s vaping devices including tanks, cylinders, posts, threads, mouthpieces, coils, atomizers, battery terminals, etc., are prone to corrosion under the right pH and thermal conditions, potentially resulting in metal particulates ending up in the vape liquid (12). For example, some of the most common materials being used in the manufacturing of these components include glass (Si, Na, Al, B), plastic (Ca, Zn, Si, Mg), stainless steel (Fe, Cr, Ni, Co, Mn), nichrome (Ni, Cr), kanthal (Fe, Cr, Al), brass (Cu, Zn, Pb) ceramic (Si, Al, Ca, B, Zr), and solder (Sn, Pb). Additionally, most vaping devices have variable voltages, allowing the user to select different vaping temperatures, based on the desired therapeutic or psychoactive effect. This means that if a higher temperature is selected, the amount and number of corroded metal particles being aerosolized could be significantly higher.
There have been very few studies carried out to understand if any of those metal particles end up in the aerosol at elevated vaping temperatures, because it is such a challenging analysis which requires a high level of operator expertise to trap and collect the aerosol containing mixtures of cannabinoids, terpenes, diluent oils, and other hydrophobic liquids in the vape tank and introduce them into the ICP-MS for analysis. It might be assumed that the high temperature vaping process of up to 600 °C would be the logical reason for corrosion of the metallic components. However, that may not necessarily be the case. A recent study by researchers at the National Research Council of Canada took 20 legal and 20 illicit unused THC vape cartridges and characterized them for a panel of 10 elemental contaminants including Cu, Zn, Pb, Fe, Cr, Ni, Mn, As, Cd, Hg (13). The legal ones were purchased at a local vape store, while the illicit ones were obtained from local law enforcement agencies. It’s also important to mention that none of the cartridges were older than eight months. However, what the researchers uncovered was quite revealing. Using ICP-MS they found high levels of all 10 toxic metals in the vape carts, which is not surprising.However, what they also discovered was variable sized particles dispersed throughout the liquid in a heterogenous manner. Moreover, on further investigation using SEM coupled with energy dispersive XRF (EDS), they confirmed these in fact were metal particles suspended in the THC liquid. It appeared the corrosion had likely taken place while the vape carts were on retailers’ shelves with the older devices showing higher levels of metal contaminants. To exemplify this, Figure 1 is a bar graph summary of the results for all 20 of the legal and 20 of the illegal vape samples.
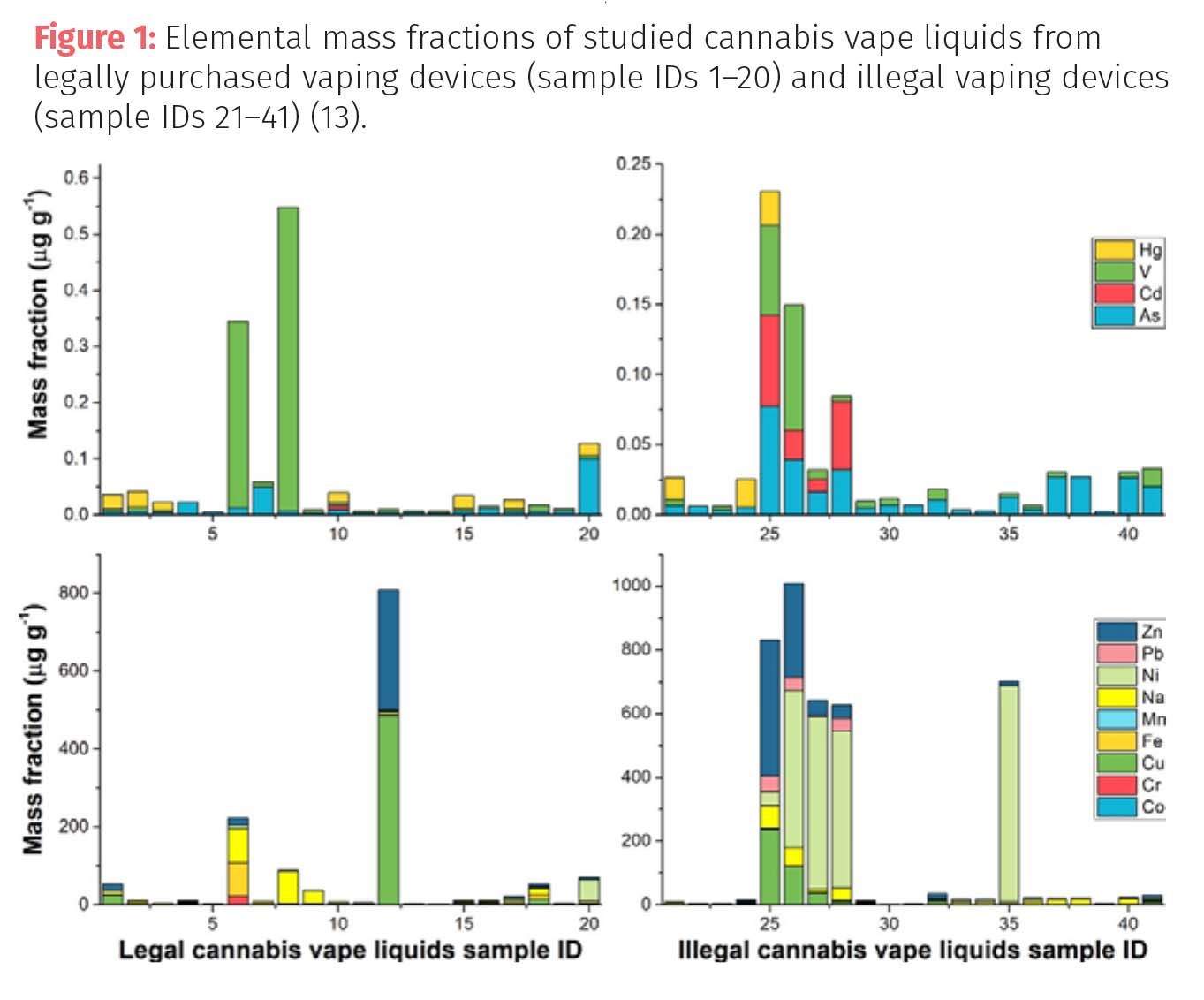
It can be seen that even though the big four heavy metals were generally below the regulated limits in many of the samples with the exception for lead, the other unregulated metals were in the hundreds of ppm, which again would escape the scrutiny of most state regulators. It’s also worth noting that lead in many of the samples was as high as 50 ppm in the illegal vapes, which suggests that it was either corrosion of the brass coil/wire or the lead-based solder of the battery terminals. On further investigation, using laser ablation ICP-MS, they found extremely high levels of Si, Na and Al in some of the particles, which perhaps infers that there could have been delamination of the glass tanks, resulting in minute particles of glass shards floating around in the vaping liquid. So, it asks the question, what type of glass components and metal alloys are used in the manufacturing of vape carts and pods and are they of the highest purity for this type of application? And in particular, are they up to strict quality standards for manufacturing drug products? Let’s take a closer look at how these types of materials are tested in the pharmaceutical industry.
Suitability of Glass
The suitability of glass for storage of corrosive chemicals used in the pharmaceutical industry was recently highlighted by Daniel Haines, PhD, Head of Pharma Services, North America,Schott AG and co-workers in the Journal of Pharmaceutical Science and Technology (14). Glass is by far the most common material used for pharmaceutical containers because of its chemical inertness and the excellent container closure properties. Nevertheless, the interaction with the drug product and various chemicals used in the manufacturing process can alter the interior glass surface and generate adverse effects. In this study, the chemical durability of borosilicate glass (Type 1 tubular and Type 1 molded glass), and molded soda lime glass (Type 2) vials were assessed using different corrosive solutions including citrates, phosphates, and chlorides defined in USP Chapter <1660>, Evaluation of the Inner Surface Durability of Glass Containers (15).
The suitability of the three different kinds of glass vails were evaluated for chemical attack based on differences in the interior glass surface using scanning SEM, the number and concentration of elements leached into solution, changes in the solution pH and visual inspection of suspended particles. The observed chemical durability differences are directly related to a combination of the glass composition, manufacturing, and processing conditions. While all three types behaved similarly when filled with ultrapure H2O, significant differences were observed when the vials were filled with a more aggressive solutions such as 15% potassium chloride (KCl).
The study is recommended reading, but in summarizing the findings, it was found that with the more aggressive solutions, the tubular borosilicate glass containers were more durable than the molded glass variety, while the soda glass was the least durable of the three types, with significantly more reactive sites and discoloration. Table III shows one dataset from the study—concentrations of the major elements in the three different types of glass by inductively coupled plasma - optical emission spectrometry (ICP-OES) over an extended period of time at elevated temperatures, emphasizing that the tubular borosilicate glass is slightly more corrosion-resistant than the molded variety, with the molded soda glass faring the worst. This is reasonable to expect based on the reduced chemical durability of soda lime compositions and their known susceptibility for dissolution and surface cracking in the presence of corrosive solutions.
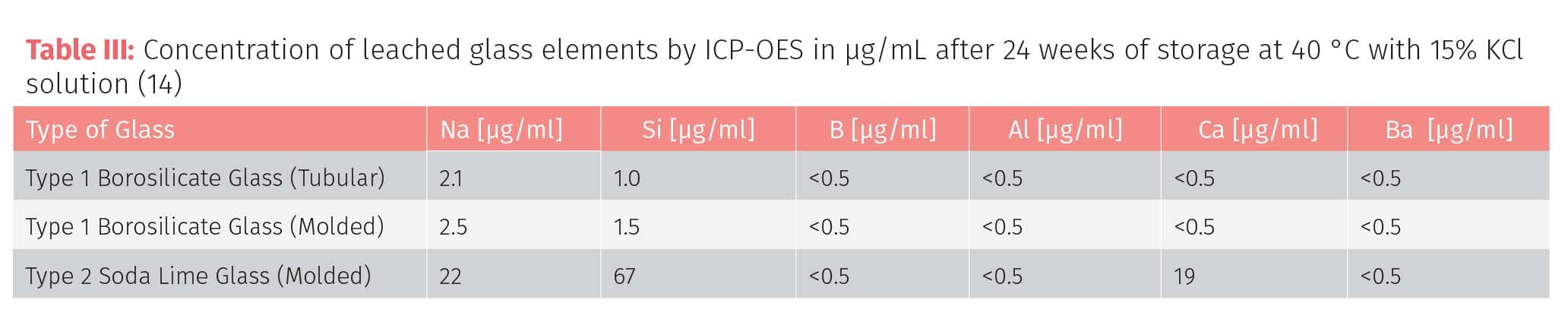
The overall conclusion of the study was that these factors should be given prime considerations when selecting containers and vials for drugs with active ingredients and also for excipients and water-based diluents with high levels of salt components. For that reason, soda glass would not be a good choice for parenteral applications for intravenous drug delivery.
This study could potentially have serious implications for the cannabis industry where glass is one of the most widely used materials for storage of cannabis extracts, oils, and diluent oils, and often for the equipment used in the cannabinoid extraction and purification process. So, it’s a very pertinent question to ask whether the optimum grades of glass are being used for the different applications including distillation vessels, storage containers, dropper bottles, consumer delivery devices such as, bongs, vape carts, etc., particularly if the application uses elevated temperatures. The limited evidence in the public domain, of ensuring the purity of glass used in vape carts, suggests that this is probably not the case (16). It’s worth noting that the pharmaceutical industry also has strict quality control procedures for plastic materials, which although different from assessing glass, is equally as stringent to ensure there is minimal contamination of drug raw materials, ingredients, and products (17).
Use of Stainless-Steel Equipment
Stainless steel is widely used in many areas of the cannabis industry, including cutting, and grinding equipment, extraction/processing vessels, mixing tanks, storage containers, screens, vaping delivery devices and much more. It is probably the most widely used material in the preparation, processing, and manufacturing of cannabis consumer products. However, because cannabis and hemp are hyper-accumulating plants, which naturally absorb metals from the soil, even surfaces, and materials they are in contact with, there needs to be careful consideration to the purity and quality of the grades of stainless steel used.
This has been exemplified by recent reports of high chromium levels in cannabis flower and biomass samples, which have been attributed to a combination of cutting with stainless steel shears and/or grinding with stainless steel blades (18). Normally this might escape the scrutiny of most state regulators, because the majority of states only require the big four heavy metals (Pb, Cd, As, Hg) to meet compliance. However, states that include Cr and/or Ni in their panel of regulated heavy metals would immediately flag this as being a potential problem, especially if the cannabis material was intended for vaping applications, where the inhalation action limits are significantly lower than for orally delivered products.
It is therefore worth understanding what types of stainless-steel are used in the cannabis industry, because it is such a common material found in many areas of the production process. As there are a multitude of types and grades of stainless steels available, with different physical and chemical properties, it is critically important that the optimum quality is used to ensure they do not contaminate any of the products they are in contact with.
We should therefore learn from the pharmaceutical industry, where using the wrong grade of stainless steel can result in premature oxidation of the surface producing corrosion and potential failure of the equipment. This could have a catastrophic impact on the cannabis manufacturing process not only for the stainless-steel cutting or grinding equipment used to prepare the cannabis plant material for cannabinoid extraction, but also from vessels used for extraction, purification, storage, and delivery purposes (19).
Future Directions and Final Thoughts
In the life cycle of the cannabis and hemp industry, it is not obvious where the heavy metal regulatory landscape will eventually end up. Clearly the big four toxic metals as required by the vast majority of US jurisdictions are not enough, but what is a realistic panel that reflects the real world of cannabinoid production? New York with eight elements and Michigan with seven, including inorganic arsenic are leading the charge with a few other states like Maryland and Missouri adding chromium to the big four. But it’s clear from the recent study carried out by the FDA’s Botanical Review Team (20), that they view the efforts of the states to regulate heavy metals as being inadequate. However, recent activity from the USP has sent signals to the industry that if they want to produce CBD as an Active Pharmaceutical Ingredient (API) for use as a federally approved drug formulation, they are most likely going to have to monitor additional elemental contaminants to meet the quality attributes. And it’s encouraging that standards organizations are developing standardized methods to meet these demands with National Institute of Standards and Technology (NIST) developing a 13-toxic element hemp reference material (10 assigned, three informational) and ASTM publishing an ICP-MS standard method for up to 23 different elemental contaminants in a wide variety of cannabis and hemp related matrices.
In conclusion, I believe that the current state-based regulatory system is unsustainable in the long term, as there is very little incentive to expand the list of analytes for fear of increasing the cost of testing and impacting revenue. It’s clear from the growing evidence in the public domain that it should be only a matter of time before the cannabis industry moves beyond the big four heavy metals and has an expanded regulated panel that is more in line with the pharmaceutical industry, and better reflects the real world of cultivation and production and most importantly, consumers’ safety concerns. However, an added twist along the way could be the significant amounts of synthetic cannabinoids such as delta-8 THC and THC-O-acetate (THC-O) that are being synthesized by “garage chemists” from the glut and over production of CBD products (20). The manufacturing process involves using organic solvents, concentrated mineral acids, and catalysts, such as, platinum group metals and zeolites (minerals containing aluminum and silicates) at high temperatures. The result is illegal and unregulated synthetic cannabinoids flooding the marketplace, which contain extremely high levels of toxic metals (21). As responsible members of this rapidly growing cannabis community, we all have a part to play in trying to better understand sources of contamination in the production of cannabinoids before consumer safety is severely compromised.
Further Reading:
- Nelson, J.; Jones, C.; Heckle, S.; Anderson, L. Determination of Heavy Metals In A Variety of Cannabis and Cannabis-Derived Products, First Action, Journal of AOAC INTERNATIONAL, 2002, 105(6), 1640-1651, https://doi.org/10.1093/jaoacint/qsab173.
- ASTM D8469-22: Standard Test Method for Analysis of Multiple Elements in Cannabis Matrices by Inductively Coupled Plasma Mass Spectrometry (ICP-MS), ASTM D37 Standards Committee, 2022, https://www.astm.org/d8469-22.html.
- Barber, C.A. ; Bryan-Sallee, C.E. ; Burdette, C.Q.;Kotoski, S P.; Phillips, M.M.; Wilson, W.B.; Wood, L.J., Cannabis Laboratory Quality Assurance Program: Exercise 2 - Toxic Elements, NIST Final Internal Report (NIST IR 8452),2022, https://nvlpubs.nist.gov/nistpubs/ir/2022/NIST.IR.8452.pdf.
- ASTM-E691 Standard Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method, Research Report No. RR:D37-2001, 2023, https://www.document-center.com/standards/show/ASTM-E691.
- Symposium on Measurement of Contaminants in Cannabis and Hemp Consumer Products, ASTM D37 Committee on Cannabis Standards, Symposium Chairs: Robert Thomas and Cary Black, 2023, http://www.scientificsolutions1.com/d37%20contaminants%20symposium%20program-1.pdf
- Macaluso, F.; Goldman, S.; James, K.A.; and Van Dyke, M., Preliminary Levels and Distribution of Health-Relevant Heavy Metals in Commercially Grown Cannabis and Hemp, Journal of Testing and Evaluation, Cannabis Contaminants Symposium Proceedings (in production), ASTM International, https://www.astm.org/products-services/standards-and-publications/journal-of-testing-and-evaluation.html#about.
- Thomas, R.J., The Role of ICP-MS in Understanding the Toxicological Link Between Lead Contamination in Cannabis and Hemp Products and Human Disease – Part 1 and 2, Analytical Cannabis, 2021, http://www.scientificsolutions1.com/the-role-of-icp-ms-in-understanding-the-toxicological-link-between-lead-contamination-in-cannabis-313507.pdf.
- Foran, A.; Slater, L.; Walton, M.; Thomas, R.; Gluodenis, T., Profiling Trace Metals of Toxicological Concern in Commercially CBD Tincture Oils, American Journal of Biomedical and Life Sciences, 2023; 11(3): 41-46, http://www.sciencepublishinggroup.com/j/ajbls.
- U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Toxicological Profile for Tin, 2005. https://www.atsdr.cdc.gov/toxprofiles/tp55.pdf.
- Hemp Extract and Heavy Metals FAQ, Florida Department of Agriculture and Consumer Services Division of Food Safety, https://ccmedia.fdacs.gov/content/download/91911/file/hemp-
- Wright, D.; Jarvie, M. M.; Southwell, B.; Kincaid, C.; Westrick, J.; Perera, S. S.; Edwards, D.; Cody, R. B., Elemental composition of commercially available cannabis rolling papers, 2024, https://pubs.acs.org/doi/10.1021/acsomega.3c09580.
- Thomas, R.J., The Challenges of Measuring Heavy Metal Contaminants in Cannabis Vaping Aerosols, Analytical Cannabis, 2021, http://www.scientificsolutions1.com/the-challenges-of-measuring-heavy-metal-contaminants-in-cannabis-vaping-aerosols-313041.pdf.
- Gajdosechova, Z.; Marleau-Gillette, J.; Turnbull, M.J.; Petts, D.C.; Jackson, S.E.; Cabecinha, A.; Abramovici, H.; Waye, A.; and Melanson, J.E., Evidence That Metal Particles in Cannabis Vape Liquids Limit Measurement Reproducibility, ACS Omega, 2022, 7, 42783-42792, DOI: 10.1021/acsomega.2c03797
- Haines, D.; Gober-Mangan, E.; Klause, M.; and Rothhaar, U., Comparative Predictive Glass Delamination Study Tubular Borosilicate vs Moulded Borosilicate and Soda Lime 20 ml Vials, Pharmaceutical Science and Technology, 2022, 84(8), 1012, https://www.researchgate.net/publication/363315970_Comparative_Predictive_Glass_Delamination_Study_Tubular_Borosilicate_vs_Moulded_Borosilicate_and_Soda_Lime_20_ml_Vials.
- USP Chapter <1660>, Evaluation of the Inner Surface Durability of Glass Containers, USP,2012, https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/c1660.pdf.
- Thomas, R., What the Cannabis Industry Should Know About Glass, Analytical Cannabis, 2023, http://www.scientificsolutions1.com/what%20the%20cannabis%20industry%20should%20know%20about%20glass.pdf
- Thomas, R., What the Cannabis Industry Should Know About Plastic, Analytical Cannabis, 2023, http://www.scientificsolutions1.com/what%20the%20cannabis%20industry%20should%20know%20about%20plastic%20.pdf
- Donovan, D., Maryland Cannabis Regulators Warn Contamination Risk as They Expand Tests for Heavy Metals, Baltimore Sun, 2019, https://www.baltimoresun.com/health/marijuana/bs-md-cannabis-heavy-metals-20190605-story.html.
- Thomas, R., What the Cannabis Industry Should Know About Stainless Steel, Analytical Cannabis, 2022, http://www.scientificsolutions1.com/what%20the%20cannabis%20industry%20should%20know%20about%20stainless%20steel.pdf
- Pruyn, S.A.; Wang, Q.; Wu, C.G.; and Taylor, C.L., Quality Standards in State Programs Permitting Cannabis for Medical Uses, Cannabis and Cannabinoid Research, 2022, https://doi.org/10.1089/can.2021.0164
- Thomas, R., PittCon 2023’s Vaping Symposium Confirms the Cannabis Industry Still Has Safety Concerns About Vaping Devices, Analytical Cannabis, 2023, http://www.scientificsolutions1.com/pittcon%202023%E2%80%99s%20vaping%20symposium%20confirms%20the%20cannabis%20industry%20still%20has%20safety%20concerns%20about%20vaping%20devices.pdf.
- The Unregulated Distribution And Sale Of Consumer Products Marketed As Delta-8 THC, US Cannabis Council, 2021, https://irp.cdn-website.com/6531d7ca/files/uploaded/USCC%20Delta-8%20Kit.pdf.
About the Guest Columnist

Robert (Rob) Thomas is the principal scientist at Scientific Solutions, a consulting company that serves the educational needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for almost 50 years, including 24 years for a manufacturer of atomic spectroscopic instrumentation. Rob has written over 100 technical publications, including a 15-part tutorial series entitled, A Beginner’s Guide to ICP-MS. He is also the editor and frequent contributor of the "Atomic Perspectives" column in Spectroscopy magazine, as well as serving on the editorial advisory board of Technology Networks. In addition, Rob has authored six textbooks on the fundamental principles and applications of ICP-MS. His most recent book is entitled A Practical Guide to ICP-MS and Other AS Techniques, which was published in September 2023. Rob has an advanced degree in analytical chemistry from the University of Wales, UK, and is also a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem).
How to Cite this Article
Thomas, R. Understanding Sources of Heavy Metals in Cannabis and Hemp Consumer Products, Part II: Is the Fractured Nature of State-based Regulations Ignoring the Evidence? Cannabis Science and Technology, 2024, 7(4), 22-29.

Newsletter
Unlock the latest breakthroughs in cannabis science—subscribe now to get expert insights, research, and industry updates delivered to your inbox.
House Budget Bill Amendment Could End Veterans Affairs Medical Cannabis Ban
July 14th 2025The “Big, Beautiful Bill” signed recently into law by President Donald Trump, includes an amendment that may end the medical cannabis ban in the VA which has prevented doctors from recommending the plant to their patients.